
To implement the newly amended “Drug Administration Law of the People's Republic of China” and “Vaccine Administration Law of the People's Republic of China”, and to further strengthen drug supervision and administration, ensure medication safety of people and promote high-quality development of pharmaceutical industry, NMPA released, on May 9, 2022, new Draft Regulations for Implementation of the Drug Administration Law of the People's Republic of China soliciting public opinions (Draft Regulations), in which among others, regulatory exclusivity of originator drugs is expanded as compared to the current rules. The deadline of providing feedback is June 9, 2022.
The Chinese version of the post may be found:
https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjyp/20220509222233134.html).
Overall, the intellectual property (IP)-related provisions of the Draft Regulations involve three aspects: regulatory exclusivity, patent linkage and patent compulsory licensing. An English translation of these provisions is attached at the end for your reference. Below please find our brief summary and comments.
Regulatory exclusivity
According to the current regulatory exclusivity provisions which have not changed for 20 years since they were created in the Regulations for Implementation of the Drug Administration Law in 2002, regulatory exclusivity is limited to 6 years data exclusivity for a drug comprising new chemical entity (NCE) and 3-5 years post-marketing surveillance period for certain drugs. Under the new Draft Regulations, however, regulatory exclusivity is expanded to certain drugs (6 years data exclusivity), pediatric drugs (maximum of 12 months market exclusivity), orphan drugs (maximum of 7 years market exclusivity) and the first successful patent challenger (12 months market exclusivity), which are also different from those in the “Implementing Provisions on Protection of Drug Trial Data (draft)” published by NMPA in April 2018 as we reported before.
The regulatory exclusivity provisions in the Draft Regulations are as follows.
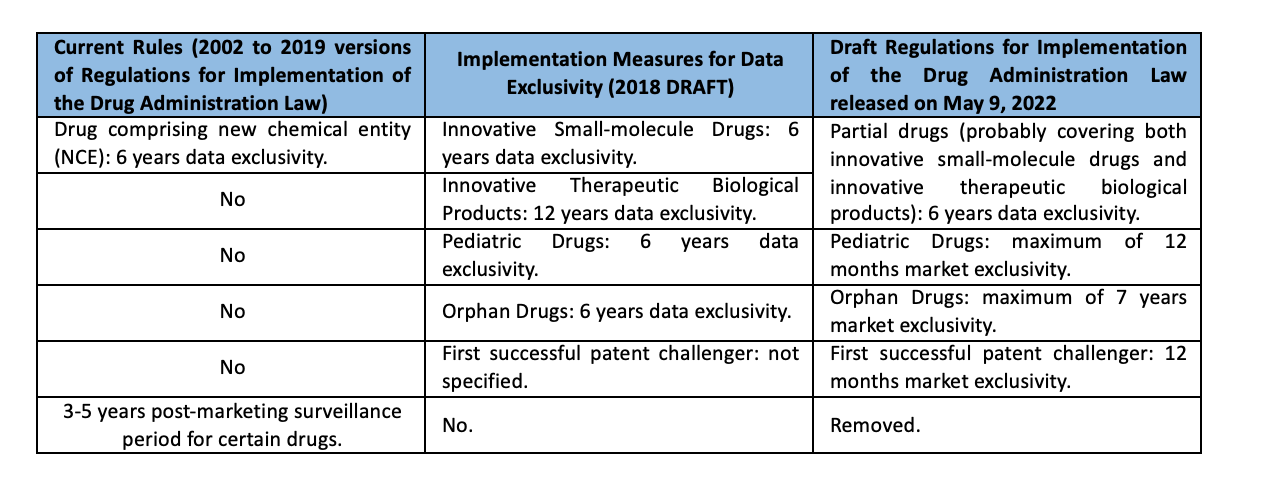
As in the hierarchy of laws, the Draft Regulations for Implementation of the Drug Administration Law is at a higher level than the Implementation Measures for Data Exclusivity, it is expected that the legislation of the Implementation Measures for Data Exclusivity which contain more details of regulatory exclusivity will be expedited as well.
Patent linkage
In addition, in the present Draft Regulations, several general provisions regarding patent linkage were introduced, which are in line with the amended Patent Law and are essentially the same as those in Measures for Implementation of Early Resolution Mechanisms for Drug Patent Disputes as we reported in 2021.
Compulsory licensing
The Draft Regulations also add an article on patent compulsory licensing, stating that for the purposes of public health or where a national emergency occurs, the patent office may grant patent compulsory license to exploit drug patents according to a provision which was introduced in the Patent Law in 2008. The implication of this article remains to be seen despite the fact that China has never granted any patent compulsory license.
Regulations for the Implementation of the Drug Administration Law
of the People's Republic of China
(Draft Amendment for Comments)
Intellectual Property Part
1. Articles on regulatory exclusivity
Article 40 【Data protection】 The State protects undisclosed trial data and other data of some drugs approved for marketing, and any person other than the drug marketing authorization holder may not make unfair commercial use of the said undisclosed trial data and other data.
Within six years from the date when a drug marketing authorization holder obtains the drug registration certificate, if any other applicant uses the data mentioned in the preceding paragraph to apply for approval for marketing of a drug without permission of the drug marketing authorization holder, the medical products administration department under the State Council shall not grant the approval, except that the other applicant submits data acquired by itself.
The medical products administration department shall not disclose the data set forth in the first paragraph of this Article except
(1) for the need of public interests; or
(2) where steps have been taken to ensure that the data are protected against unfair commercial use.
Article 178 【Penalties for data disclosure】Where the medical products administration department and its staff members, in violation of regulations, release undisclosed trial data or other data, thus resulting in losses to the applicant, the medical products administration department shall be liable for compensation in accordance with law. After compensating the losses, the medical products administration department shall order the staff members who disclose the said data in purpose or have serious negligence to partially or fully bear the compensation and shall also impose administrative sanctions on those who are directly responsible therefor.
Article 28 【Pediatric drugs】paragraph 2: For a new variety, dosage form and strength exclusively for children, and a drug for increasing an indication or usage and dosage for children, which are the first to have been approved for marketing, a market exclusivity period of no more than 12 months shall be granted, during which the marketing of the same variety shall not be approved.
Article 29 【Rare diseases】paragraph 2: For a new drug for rare diseases that has been approved for marketing, a market exclusivity period of no more than 7 years shall be granted under the condition that the drug marketing authorization holder promises to guarantee drug supply, during which the marketing of the same variety shall not be approved. If the drug marketing authorization holder fails to fulfill the supply guarantee promise, the market exclusivity period shall be terminated.
Article 39 【Promoting the development of generic drugs】 The State encourages the development of generic drugs. For the chemical generic drug that is the first to have successful challenge to the patent and is the first to have been approved for marketing, a market exclusivity period shall be granted. The medical products administration department under the State Council shall not approve the marketing of generic drugs of the same variety within 12 months from the date of approval of the drug, except for those who successfully jointly challenge the patent. The market exclusivity period does not exceed the original patent right term of the challenged drug.
2. Articles on patent linkage
Article 38 【Patent Linkage】 Where a patent right is disputed during drug registration application, the party may take legal action before a people's court or file a request for administrative adjudication with the patent administration department under the State Council, during which the drug technical review is not stopped. For a chemical drug that has passed the technical review, the medical products administration department under the State Council shall make a decision as to whether the marketing of the drug is approved, in accordance with the effective judgment, ruling or mediation certification from the people's court or the administrative adjudication from the patent administration department under the State Council; if no effective judgment, ruling, mediation certification or administrative adjudication is obtained beyond a certain period, the medical products administration department under the State Council can approve the marketing of the drug.
The medical products administration department under the State Council shall establish the registration platform of drug patent information. Drug registration applicants and drug marketing authorization holders shall register related drug patent information according to the regulation, and specify the related drug patents involved and the status thereof.
The drug registration applicants and the drug marketing authorization holders shall be responsible for the authenticity, accuracy and completeness of the information registered by them.
3. Articles on patent compulsory licensing
Article 121 【Patent compulsory licensing】 For the purposes of public health or where a national emergency occurs, the Health Administration Department under the State Council may make suggestions of patent compulsory licensing according to the disease diagnosis and treatment needs. An enterprise with corresponding conditions may file an application with the Patent Administration Department under the State Council, and the Patent Administration Department under the State Council may grant a compulsory license to exploit a patent granted for a drug in accordance with the provisions of Article 54 of Patent Law of the People's Republic of China. The Medical Products Administration Department under the State Council shall grant prioritized review and approval of the drug for which a patent compulsory license is granted according to regulations.
Note:
Article 54 of the currently effective Patent Law of China reads,
Where a national emergency or any extraordinary state of affairs occurs, or where the public interest so requires, the Patent Administration Department under the State Council may grant a compulsory license to exploit the patent for invention or utility model.